How helpful is data that isn’t statistically sound, clean, and reliable? Not very. But, thanks to clinical data management, it’s possible to ensure that your data is up to par.

The medical research process includes the production of a lot of data, and in order to ensure that it’s used in the most effective manner possible, you’ll need a sound data management strategy.
So, what is clinical data management, and what does it look like in practice? In this post, we’ll answer these questions and share the best clinical data management tools, so you’re fully equipped with the skills and tools necessary to succeed.
- What is clinical data management?
- Why is clinical data management necessary?
- Main Objectives of Clinical Data Management
- What are clinical data management tools?
- What are the stages of a clinical data management cycle?
What is clinical data management?
Clinical data management (CDM) refers to the collection and management process of research data in accordance with regulatory standards. These standards ensure quality information that is error-free and complete. It must adhere to federal, state, and local guidelines.
Because clinical data management handles information from clinical trials, it’s essential that software systems, processes, training, procedures, databases, and protocols all support the proper collection, cleaning, and management of trial data.
CDM originated due to demands by regulatory authorities and the pharmaceutical industry. While the pharmaceutical industry wants to create and release products as rapidly as possible, regulatory bodies decided to enact rules of their own. They required that companies meet certain standards when collecting the data that’s used in the process of drug evaluation.
We can’t discuss CDM without mentioning two standards: the Clinical Data Acquisition Standards Harmonization (CDASH) and the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG). The Clinical Data Interchange Standards Consortium (CDISC) is to thank for these standards. (Can you tell they like their long titles, and acronyms?)
Why is clinical data management necessary?
Clinical data management is essential as it ensures the data that is produced through a clinical trial is high-quality. When performed properly, clinical data management results in a dataset that is reliable, accurate, and analysis-ready when the study concludes.
Furthermore, CDM offers essential support for the evaluation process of regulated goods including cosmetics, food, medical devices, and pharmaceuticals. To guarantee that the products you’re getting are safe and will work as they should, adherence to regulatory standards is necessary.
Here are some of the benefits clinical data management offers:
- Data won’t get lost.
- Development is expedited.
- There is greater security.
- Data quality is guaranteed.
- Costs are reduced.
- Data collection is accurate and complete.
- The data is correctly formatted for optimal usability.
- The trial is accurately represented in the database.
- There’s a clean dataset available for reporting.
Main Objectives of Clinical Data Management
While there are various benefits associated with clinical data collection, its primary goals can be distilled into three main objectives. Let’s dive deeper into these.
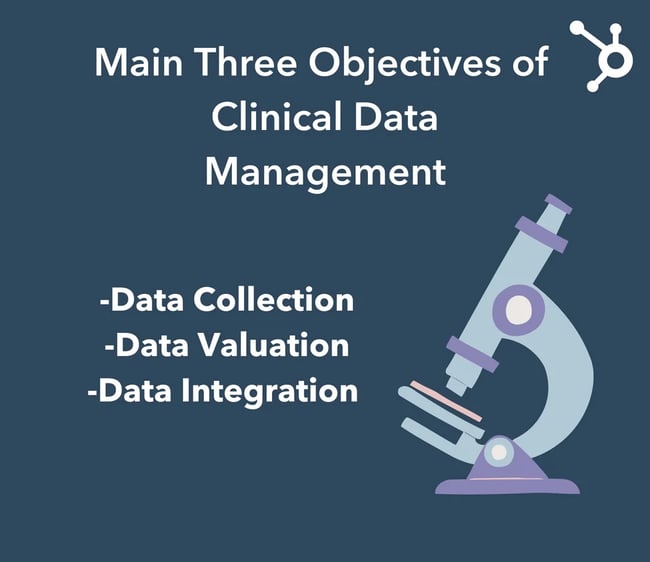
1. Data Collection
Regardless of whether the data is in paper form or available electronically, clinical data management helps ensure that information is properly collected. Furthermore, correctly collecting and storing the data ensures that when it is needed later, it will be easily accessible.
2. Data Valuation (of data and system)
Valuation is the appraisal and estimate of an item’s worth. This includes manual review for additional oversight, plus user acceptance testing (UAT), programming completed with edit checks in place, and quality controls. The aim of this is to identify how useful the data is.
3. Data Integration
Lastly, data integration allows you to put all of the data into a singular database. Having all your information in one place supports correctness and consistency. Similar to data collection, this also simplifies the process of accessing the data at a later time.
What are clinical data management tools?
CDM is made possible with specialized software applications. These applications help users conduct audits to detect discrepancies and minimize them — even in large, complex clinical trials. Some clinical data management tools you may be familiar with are Oracle Clinical, Clintrial, Rave, eClinical suite, and Macro.
With trials conducted in medical centers that produce massive amounts of data, it’s especially critical that effective clinical data management systems are used. These systems can be customized and often are by huge international pharmaceutical companies that want to address their specific requirements.
However, there are various clinical data management tools available. Some highly effective options are even open-source tools like OpenClinica, PhOSCo, open CDMS, and TrailDB.
What are the stages of a clinical data management cycle?
Now that you know more about the tools needed for successful clinical data management, let’s dive into the cycle that is essential to keeping your data safe and secure.
1. Prepare.
First, collect all of your necessary forms — whether those be electronic or paper. Then, review your plan and ensure your database is ready to receive information. The more organized you are at this phase of the process, the more seamless the rest of it will be.
2. Collect data.
Now, you can begin the process of collecting your data. Continue to gather data throughout your study. Remember: accuracy is first and foremost. It’s not a bad idea to check in periodically to make sure that your data is accurate.
3. Ensure accuracy.
Make sure that the tools you’re using, your plan itself, and the data collected meet regulatory requirements. Data quality is a top priority, so if there are any discrepancies, the sooner you can figure it out the better.
4. Keep track and preserve your data.
It’s time to keep track of your data. Make sure that you’re monitoring it for potential issues or risks that may pop up. This is also the right time to think about what you can do to preserve your data’s quality.
5. Integrate your data.
You’ve monitored your data for issues, and preserved its integrity. Now, it’s time to map out datasets and information together. Consistency is critical — as it has been throughout the entirety of the process.
6. Analyze the outcomes.
Because your data is clean and consistent, you can now use it to analyze the outcome and feel secure about its accuracy. That brings us to our next point, that your clinical data management cycle isn’t done once you’ve analyzed the outcome. Now, it’s time to make sure your data is protected.
7. Protect your data.
After completing the clinical data management cycle, take time to secure your database so no information is misplaced or erroneously edited.
Clinical Data Management Ensures Data Integrity
The main purpose of clinical data management is to ensure data integrity. If you follow the steps outlined and utilize the clinical data management tools to their fullest potential, you’ll obtain data that’s able to be analyzed and understood.